Which of the Following Is a Characteristic of Sodium Ion
The pump moves three sodium ions out of a cell and two potassium ions into a cell and generates an ATP in each cycle. C The pump protein undergoes a conformational change.
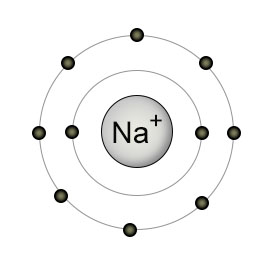
For example a sodium atom Na has a single electron in its valence shell surrounding 2 stable filled inner shells of 2 and 8 electrons.
. Sodium ion Na has 10 electrons. The rate is proportional to the concentration of sodium cyanide C. 59 Which of the following characterizes the sodium-potassium pump.
It requires no energy. The atomic number of Oxygen O2 is 8. The presence of sodium ions in a salt can be determined by the color of a flame Na turns flame yellow.
Glass electrodes can be used to measure. Question 13 The high concentration of protons in the inner mitochondrial space relative to the mitochondrial matrix. Since the sodium-ion battery has no over-discharge characteristics the sodium-ion battery is allowed to discharge to zero volts.
The number of neutrons in this isotope of sodium is A 11. 26 Consider an isotope of sodium with a mass number of 25. Properties of Acids and Bases.
Regulating the transport of ions across cell membranes. An atom of sodium Na donates one of its electrons to an atom of chlorine Cl in a chemical reaction and the resulting positive ion Na and negative ion Cl form a stable ionic compound sodium chloride. A positive test for a base occurs when.
The rate is proportional to the concentration of R-2-bromobutane. It is an antiport. Red litmus remains red.
50 SS S A 1 18 protons 50 neutrons 118 electrons. Its octet is complete so the number of valence electrons is zero. The reaction proceeds with inversion of configuration B.
A Sodium b Hydrogen c Nitrogen d Chlorine. Blue litmus remains red. A ATP B oxygen C Na D glucose E light Answer.
It requires no energy Question. In glass membrane electrode the glass containing 11 Na 2 O 18 Al 2 O 3 71 SiO 2 is highly sensitive to which of the following ions. Check all that apply.
For a particular cell the concentration of sucrose is 10 mM on the inside of the cell and 05 mM on the outside whereas the concentration of sodium ions Na is 05 mM on the inside of the cell and 10 mM on the outside. The electronic configuration of sodium ion is 1s2 2s2 2p6 3s0. It is an antiport.
Up to 24 cash back A. All elements along a period have the same number of electron shell while all elements down a group have the same number of electrons in the outermost electron shell. It pumps 3 sodium ions out of a cell and 2 potassium ions into a cell simultaneously.
A The sodium ions are moving down their electrochemical gradient while glucose is moving up B Glucose is entering the cell along its concentration gradient C Sodium ions can move down their electrochemical gradient through the cotransporter whether or not glucose is. E A B and C are all correct. It is an antiport.
Which of the following molecules can pass through the plasma membrane by simple diffusion. A Sodium ions are pumped out of a cell against their gradient. Which of the following statements is not true regarding the SN2 reaction of R-2-bromobutane with sodium cyanide.
The distribution of sodium and potassium ions across the membrane of an axon is maintained by A. Common table salt based on this ionic bond. Select one or more.
Metallic sodium is usually stored under a layer of kerosene as it has high chemical reactivity Na can react violently with oxygen and air moisture even at room temperature. The electronic configuration of sodium is 1s2 2s2 2p6 3s1. Which of the following best describes the location of ions during resting potential.
B Potassium ions are pumped into a cell against their gradient. Calcium in the bodys fluids performs all of the following major roles except. In glass membrane electrode the glass containing 11 Na 2 O 18 Al 2 O 3 71 SiO 2 is highly sensitive to sodium ions.
4 Lesson 8 Periodic Table and Its Periodicity Periodic Table of Elements was arranged according to increasing atomic number from left to right along a period and down a group. D Only A and B are correct. B 27 Which of the following gives the correct numbers of protons neutrons and electrons in 118 a neutral atom of Sn.
It pumps 3 sodium ions out of a cell and 2 potassium ions into a cell simultaneously. Which of the following is NOT a characteristic of the sodium-potassium pump. Participating in blood clotting.
ASodium ion Na The atomic number of Sodium Na is 11. It is a cell membrane protein. Which of the following statements best describes characteristic activities of a sodium-potassium pump.
The pump moves three potassium ions out of a O cell and two sodium ions into a cell using energy from ATP hydrolysis. Helping maintain normal blood glucose. Since these filled shells are very stable a sodium atom tends to lose its extra electron and attain this stable configuration becoming a sodium cation in the process.
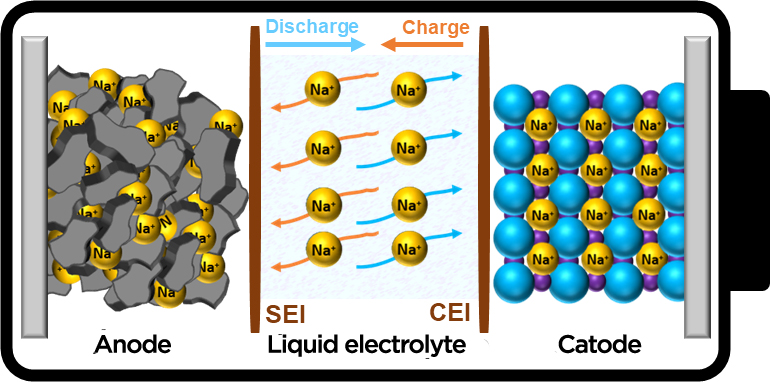
Sodium-ions do not form an alloy with aluminum and aluminum foil can be used as the current collector for the negative electrode which can further reduce the cost by about 8 and reduce the weight by about 10. A Base your answers to the questions on the following information.

Sodium Ion Batteries Towards A Sustainable Low Cost Energy Storage Technology Cic Energigune


No comments for "Which of the Following Is a Characteristic of Sodium Ion"
Post a Comment